-
- California Assembly for second time adopts same-sex marriage bill
- Pentagon’s Peter Pace out as Chair of Joint Chiefs
- HIV testing for pregnant women, newborns advances in N.J.
- Mass. gov. first to walk in Boston’s Pride parade
- Abbott Laboratories, Thailand face off in HIV-drug patent stalemate
- Navy assigns openly gay sailor Jason Knight to reserves
- National News Briefs
- World News Briefs
national
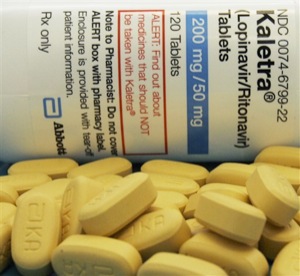
Abbott Laboratories, Thailand face off in HIV-drug patent stalemate
Standoff could determine how people in developing countries get medicine
Published Thursday, 14-Jun-2007 in issue 1016
CHICAGO (AP) – On the surface, it’s a complex patent fight spanning thousands of miles and more than a dozen time zones.
But strip away the lawyers, government officials and fleet of executives, and the standoff between Thailand and Abbott Laboratories Inc. could determine how, and if, millions of people in developing countries get medicine in the future.
Thailand’s government decided late last year to override a series of drug patents, but instead of creating a cheap solution to pay for medication for more than 500,000 Thai infected with HIV and AIDS, the military-installed government wound up embroiled in one of the most-watched patent disputes in recent years. Now it’s blacklisted from receiving Abbott’s new medications while faced with the prospect of economic sanctions.
“The stakes here are very high because many patients around the world are in need of newer drugs,” said Michael Weinstein, president of the activist organization AIDS Healthcare Foundation, which supports the Thai government’s decision.
At issue is how countries negotiate lower drug prices and how they use international trade rules to issue so-called “compulsory licenses” to scrap patents in favor of cheaper, generic medication. The debate also shows how vulnerable the pharmaceutical industry is to efforts by countries trying to override patents.
For drug companies, patents are an incentive to invest billions in future research and development and reward them for producing lifesaving products.
“This misguided focus on short-term ‘budget fixes’ could come at a far greater long-term cost, potentially limiting important incentives for research and development that are necessary to positively impact the lives of millions of patients worldwide,” Pharmaceutical Research and Manufacturers of America President Billy Tauzin said in a statement.
Industry officials also caution cheaper, generic versions can be less effective or even unsafe for patients, especially in regions of the world where drug manufacturing isn’t stringently monitored.
Health activists, meanwhile, applaud the decision, saying compulsory licenses are a way for countries to cost-effectively treat the AIDS epidemic.
The Thai action marked the first time a country used the measure for a “second-line” AIDS drug: Abbott’s billion-dollar blockbuster, Kaletra. As HIV patients survive longer, people have become increasingly resistant to older first-line drugs. That means hundreds of thousands more patients will require second-line virus-fighting medicine by 2010 – drugs that can cost 10 times more than first-line treatments.
In Thailand, where the per capita income is $2,720 year, patented Kaletra was sold for $2,200 a year before the company lowered its price to $1,700 in the fall.
Thailand’s Public Health Minister Mongkol Na Songkhla said the decision to override patents is justified because the drugs’ high cost constituted a health crisis. The country’s AIDS budget – around $112 million – was only enough to pay for medicine for one-fifth of patients. The country has hinted cancer drugs could be next.
In response, Abbott pulled its seven drug applications pending before health regulators in Thailand – including one for Aluvia, a form of Kaletra developed for use in tropical countries – cutting off the country’s 65 million residents from new medication developed by the North Chicago-based company.
|
|
Copyright © 2003-2026 Uptown Publications